Answer:
Because an empirical formula only shows the most simple ratio between elements in a compound while the molecular formula gives the actual number of each element in a certain molecule. Take the example of glucose. It has a molecular formula of
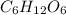 . However, the smallest ratio between the atoms are 1:2:1 respectively. So the empirical formula would be
. However, the smallest ratio between the atoms are 1:2:1 respectively. So the empirical formula would be
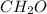 . Clearly not representing the molecular formula.
. Clearly not representing the molecular formula.
Step-by-step explanation: