Step-by-step explanation:
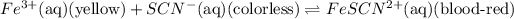
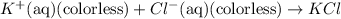
The above two reactions xcan also be written in form of single chemcial equation:
![FeCl_3+K(SCN)\rightleftharpoons KCl+[Fe(SCN)]Cl_2(blood-red)](https://img.qammunity.org/2019/formulas/chemistry/high-school/vuymbei4nwk83m74lxztidwx4zt6apl4i4.png)
1. Color of ferric chloride solution is yellow. This is due to presence of ferric ions which have yellow color in their aqueous solutions.
2. KSCN has the colorless solution. This due to potassium ion forms colorless aqueous solution.
3. On mixing, KSCN with
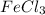 we will get blood red color solution of
we will get blood red color solution of
![[FeSCN]Cl_2](https://img.qammunity.org/2019/formulas/chemistry/high-school/ehrhqm36kf88wjax2w1kh5jvxwewleykhu.png) .
.