Answer 1: The correct answer is option (B)
Answer 2: The correct answer is option (B)
Explanation 1:
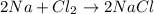
moles of NaCl =
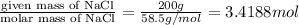
According to reaction, 2 moles NaCl are obtained from 2 moles of Na, then 3.4188 moles of NaCl are obtained from:
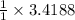 moles of Na.
moles of Na.
Mass of Na = moles of sodium × Molar mass of sodium
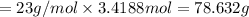
From the given option nearest answer to calculated answer is option (B) that is 78.65 g.
Explanation 2:
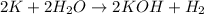
moles of potassium(K)=
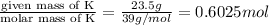
According to reaction, 2 moles of K gives 2 moles of KOH , then 0.6025 moles of K will give :
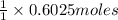 of KOH
of KOH
Mass of KOH = moles of KOH × Molar mass of KOH
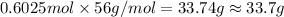
From the given option nearest answer to calculated answer is option (A) that 33.7 g.