Step-by-step explanation:
The products of chemical reaction will be as follows.
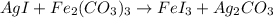
To balance the equation, we will count the number of atoms on both reactant and product sides as follows.
Number of atoms on reactant side are as follows.
- Ag = 1
- I = 1
- Fe = 2
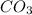 = 3
= 3
Number of atoms on product side are as follows.
- Ag = 2
- I = 3
- Fe = 1
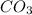 = 1
= 1
We will balance the equation by multiplying AgI by 6 on the reactant side and
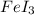 by 2 and
by 2 and
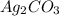 by 3 on the product side. Therefore, balanced chemical equation will be as follows.
by 3 on the product side. Therefore, balanced chemical equation will be as follows.

In this reaction, iron is replaced by silver and carbonate is replaced by iodide. Thus, it is a double displacement reaction.