Final answer:
To produce 3.33 moles of aluminium chloride, you would need 3.33 moles of aluminium metal.
Step-by-step explanation:
To find the number of moles of aluminium metal needed to produce 3.33 moles of aluminium chloride, we need to use the stoichiometry of the balanced chemical equation.
From the equation:
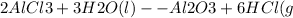 ,
,
we can see that 2 moles of AlCl3 are produced from 2 moles of aluminium.
Therefore, in order to produce 3.33 moles of AlCl3, we would need 3.33 moles of aluminium.