Answer: The correct answer is option B.
Step-by-step explanation:
- Ionic bond is formed when there is complete transfer of electrons from one element to another.
- Covalent bond is formed by the sharing of electrons between two elements.
- Metallic bond is formed when two metals combine together.
Electronic Configuration of Group VII-A :
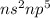
This group require 1 electron to attain stable electronic configuration.
Electronic configuration of Group I-A :
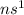
This group will loose 1 electron to attain stable electronic configuration.
So, there will be complete transfer of electron from Group VII-A to Group I-A and hence, will form ionic bond between them.
Hence, the correct option is B.