Explanation: There are 2 given isotopes of Copper.
Mass number of Isotope 1 : 63amu
Mass number of Isotope 2: 65 amu
Let the fractional abundance of isotope 1 be 'x', so the fractional abundance for Isotope 2 will be '1-x'
The average atomic number of Copper given is 63.55 amu.
So, the formula for average atomic mass is given by:
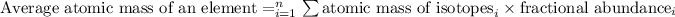
Putting values in above equation, we get:
![63.55=[(63* x)+(65* (1-x))]\\\\x=0.725](https://img.qammunity.org/2019/formulas/chemistry/middle-school/eb5zih9efycrkutzo70cu97ibdg55fmuvs.png)
The fractional abundance of Isotope 1 = 0.725
The fractional abundance of Isotope 2 = 0.275
So, the natural percentage abundance for isotope 1 = 72.5%
The natural percentage abundance for isotope 2 = 27.5%
Whenever more number of isotopes are present of an element are present, we take the average atomic mass of that element.