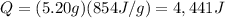Answer:
4441 J
Step-by-step explanation:
The amount of energy needed to change the ethyl alcohol from liquid to gas state is given by
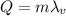
where
m = 5.20 g is the mass of the alcohol
 is the latent heat of vaporization of the substance
is the latent heat of vaporization of the substance
Substituting the numbers into the formula, we find: