Answer:- A.
 and
and
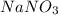
Explanations:- In general,
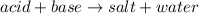
In acid-base reaction the acid gives hydrogen ion and the base gives hydroxide ion and these ions combine to form water. Rest of the ions from acid and base combine to form the salt.
Nitric acid breaks to give hydrogen ion and nitrate ion. Similarly, NaOH breaks to give sodium ion and hydroxide ion. Since, hydrogen ion and hydroxide ions form water, the sodium ion and nitrate ion forms the salt, sodium nitrate.
The reaction is shown as:
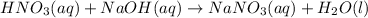
So, the right choice is A.