Answer : Two electrons balances the charges in this half reaction.
Explanation :
The given half reaction is,
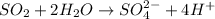
In this half reaction, there is no charges present on the left side of the reaction but on right side of the reaction, (2+) charge is present. So, two electrons (2-) are added on the right side of the reaction for balances the charge of the half reaction.
The balanced half reaction is,
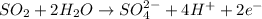
Hence, two electrons balances the charges in this half reaction.