Step-by-step explanation:
An endothermic reaction is a reaction in which the reactants absorb energy and the energy of products is more than that of reactants.
Whereas an exothermic reaction is a reaction in which the reactants have more energy as compared to products. Hence, there will be release of energy.
Therefore, when temperature is increased by
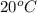 then it means we are providing more energy to the reactants. As a result, there will be release of energy. Hence, the reaction will be exothermic in nature and not endothermic.
then it means we are providing more energy to the reactants. As a result, there will be release of energy. Hence, the reaction will be exothermic in nature and not endothermic.
Thus, we can conclude that the student is incorrect because the reaction is exothermic and not endothermic.