Answer:

Step-by-step explanation:
First, convert the grams to moles, then convert moles to atoms.
1. Grams to moles
To convert from grams to moles, we use the molar mass. Phosphorus's molar mass is given to use.
- Phosphorus (P)= 30.97 g/mol
We can use this number as a fraction.
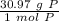
Multiply by the given number of grams (1.01)
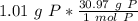
Flip the fraction so the grams of phosphorus cancel out.
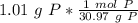
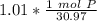
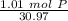
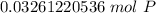
2. Convert Moles to Atoms
To convert from moles to atoms, we use Avogadro's Number.
This tells us the number of particles in 1 mole. In this case, it is atoms of phosphorus in 1 mole of phosphorus. We can create a fraction.
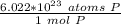
Multiply by the number of moles we calculated.
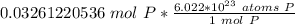
The moles of phosporus will cancel.
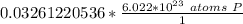
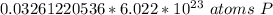
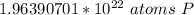
This is closest to 1.97*10²² atoms, so choice B is correct.