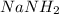Answer : The empirical formula of the compound is, (c)
Solution : Given,
Moles of sodium, Na = 0.5 moles
Moles of nitrogen, N = 0.5 moles
Moles of hydrogen, H = 1.0 moles
Divide the each moles value by the smallest number of moles of given element.
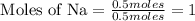

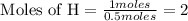
The mole ratio of the elements are,
Na : N : H = 1 : 1 : 2
The mole ratio of the element is represented by the subscripts in the empirical formula.
The empirical formula is written as,
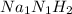 or
or
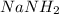
Therefore, the empirical formula of the compound is, (c)