Answer : The pressure of the gas will be, 5.285 atm
Solution : Given,
Volume of
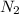 gas = 10 L
gas = 10 L
Temperature of gas =
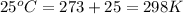
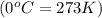
Volume of
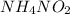 = 2.40 L
= 2.40 L
Molarity of the ammonium nitrate solution = 0.9 M
First we have to calculate the moles of ammonium nitrate.
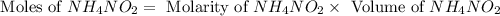
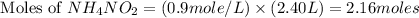
The balanced chemical reaction is,
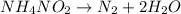
From the balanced reaction, we conclude that
1 mole of
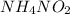 decomposes to give 1 mole of
decomposes to give 1 mole of
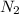 gas
gas
2.16 moles of
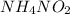 decomposes to give 2.16 moles of
decomposes to give 2.16 moles of
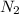 gas
gas
Now we have to calculate the pressure of the gas.
using ideal gas equation,
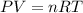
where,
P = pressure of the gas
V = volume of the gas
T= temperature of the gas
n = number of moles of gas
R = gas constant = 0.0821 Latm/moleK
Now put all the given values in ideal gas equation, we get pressure of the gas.
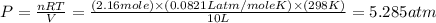
Therefore, the pressure of the gas will be, 5.285 atm