Answer : The volume of
 will be 0.4665 L
will be 0.4665 L
Solution : Given,
Initial volume = 500 ml = 0.5 L (1 L = 1000 ml)
Initial pressure = 742 torr =
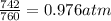
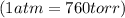
Final pressure = 795 torr =
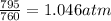
According to the Boyle's law, the pressure of the gas is inversely proportional to the volume of the gas at constant temperature.
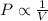
or,
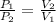
where,
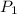 = initial pressure of the gas
= initial pressure of the gas
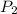 = final pressure of the gas
= final pressure of the gas
 = initial volume of the gas
= initial volume of the gas
 = final volume of the gas
= final volume of the gas
Now put all the given values in the above formula, we get
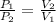
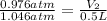
By rearranging the terms, we get the final volume of the gas.
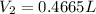
Therefore, the volume of
 will be 0.4665 L
will be 0.4665 L