Explanation: A thermochemical equation is a balanced chemical equation which require an enthalpy change.
A balanced equation is the equation in which number of atoms on reactant side is same as the number of atoms on product side.
A thermochemical equation for
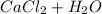 is:
is:
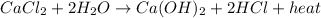
Here, heat is released in the reaction hence, it is a type of exothermic reaction.
A thermochemical equation for
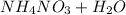 is:
is:
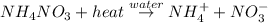
Here, heat is absorbed in the reaction hence,it is a type of endothermic reaction.