Answer : The heat given off to the surroundings will be, -155.38 KJ
Solution : Given,
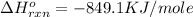
Mass of aluminium = 9.873 g
Molar mass of aluminium = 27 g/mole
First we have to calculate the moles of aluminium.
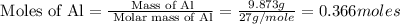
The given balanced reaction is,
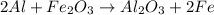
From the reaction, we conclude that
2 moles of aluminum releases heat energy = -849.1 KJ
0.366 moles of aluminium releases heat energy =
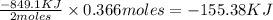
Therefore, the heat given off to the surroundings will be, -155.38 KJ