Answer:
4Na + O₂ → 2Na₂O
6.82g
Step-by-step explanation:
The combustion of sodium involves combining the element with oxygen gas:
4Na + O₂ → 2Na₂O
We have been given 5g of Na.
The balanced reaction equation is given as shown above. This reaction is also a synthesis or combination reaction.
b. Maximum amount of product that can be obtained.
Sodium is the limiting reactant and it will determine the amount of product that can be formed.
To solve this, we find the number of moles of the given sodium.
Mass of given sodium = 5g
Molar mass of Na = 23g/mol
Number of moles of Na =
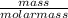
Insert the parameters and solve;
Number of moles of Na =
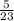 = 0.217moles
= 0.217moles
From the balanced reaction equation:
4 moles of Na will produce 2 moles of Na₂O
0.217 moles of Na will produce
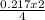 = 0.11mole of Na₂O
= 0.11mole of Na₂O
So;
Mass of Na₂O = number of moles x molar mass
Molar mass of Na₂O = 2(23) + 16 = 62g/mol
Mass of Na₂O = 0.11 x 62 = 6.82g