Answer: The name given to
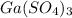 is Gallium (III) sulfate.
is Gallium (III) sulfate.
Explanation: This is an ionic compound because in aqueous solution it dissociates into its respective ions.
Naming of Ionic compounds.
- Name the cation first and then write its oxidation number in roman numerical.
- Then name the anion or polyatomic ions without writing any prefix of the number of atoms present in it.
- The name of the anion should have a suffix '-ide' like for chlorine, the name will be chloride etc..
- For polyatomic ions, the suffix used will be '-ate' like for
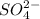 the name will be sulfate etc..
the name will be sulfate etc..
Name of the given ionic compound is Gallium (III) Sulfate.