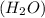Answer: Products of the reaction are lithium fluoride and water.
Step-by-step explanation: When an acid reacts with a base, it yields a salt and water. This reaction is known as neutralization reaction because acid-base neutralize each other in this reaction.
Equation for this reaction follows:

Reaction of acid (HF) and base (LiOH) follows the equation:

The products of this chemical reaction are lithium fluoride (LiF) and water