Answer: The mole ratio of D : A is 3 : 2.
Explanation: Mole ratio for a chemical reaction is the ratio of their respective stoichiometric numbers or the ratio of their moles in a chemical reaction.
For a given chemical reaction:
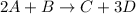
By stoichiometry,
2 moles of A is reacting with 1 mole of B to produce 1 mole of C and 3 moles of D.
So, Mole ratio of D is to A will be 3 : 2