Answer: the correct answer is option(d).
Step-by-step explanation:
Energy required ,Q= ?
Mass of water , m = 17 grams
Temperature difference or
 = 15 °C
= 15 °C
Specific heat value of water = 4.186 J/g °C
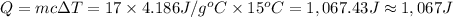
From the given value the , nearest value to to our calculated answer is 1067 J that option (b).
Hence, the correct answer is option (d).