Answer: Statement is true.
Step-by-step explanation:
Synthesis reaction is a type of reaction in which two or more chemical species combine together to result in the formation of a complex product.
The reactants in a synthesis reaction may be elements or compounds but the product will always be a compound.
For example,
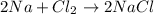 is a synthesis reaction.
is a synthesis reaction.
Thus, we can conclude that a synthesis reaction can be identified because it involves more than one reactant forming one compound as the product is a true statement.