Answer:
Option C is correct.
Atoms of oxygen exist in the products of this reaction is 6.
Explanation:
Given a chemical equation:
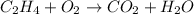
Law of conservation of mass states that it is observed in a balanced chemical equation, which is a chemical equation that shows all mass is conserved throughout the reaction.
To balance the given equation:
First start off by balancing the elements in the complex molecules first and then the single element molecules.
First, we will start with balancing the Carbons.
On the left we have 2 but on the right only 1, so we multiply the
 on the right by 2.
on the right by 2.
Next we look at the Hydrogen.
We have 4 on the left to the given equation, but only 2 on the right.
To balance them;
so, multiply
 by 2.
by 2.
then, equation become;
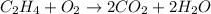 .....[1]
.....[1]
Now, looking at the right hand side we have oxygen, 4 from
 and 2 from
and 2 from
 , then we have total number of oxygen on right hand side is 6.
, then we have total number of oxygen on right hand side is 6.
In order to have 6 on left side we balanced the equation [1] multiply
 by 3.
by 3.
then the balanced given equation is:
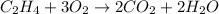
Number of oxygen atom exist =
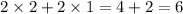
Therefore, number of atoms of oxygen exist in the products of this reaction is 6.