Answer:
Hexane.
Step-by-step explanation:
Hello!
In this case, since the general reaction of the compound C4H14 with chlorine is:
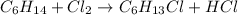
Which stands for a substitution chemical reaction in which one chlorine is able to replace one hydrogen and therefore hydrogen chloride gives off; we infer that the initial compound, C4H14, shows off the
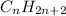 formula characteristic of alkanes; in such a way, as it has six carbon atoms, we infer it is hexane.
formula characteristic of alkanes; in such a way, as it has six carbon atoms, we infer it is hexane.
Best regards!