Answer: The charge on the atom present in oxygen family is -2.
Step-by-step explanation:
Oxygen family belongs to Group 16 of the periodic table.
The electronic configuration for this group elements is
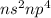
where, n is the number of periodic number.
The elements having this electronic configuration will gain 2 electrons in order to attain stable configuration.
When an atom gains electrons, it will attain negative charge. Thus, the atoms present in group 16 of the periodic table will attain -2 charge.