The question is incomplete, here is the complete question.
Which metal will form a compound with the general formula
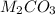 when it combines with a carbonate ion
when it combines with a carbonate ion
A. Beryllium
B. Aluminum
C. Calcium
D. Lithium
Answer: The metal that will form the given compound is lithium.
Step-by-step explanation:
We are given:
A general chemical formula of carbonate, which is
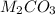
The given compound is an ionic compound.
Carbonate is a polyatomic ion having chemical formula
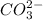
Metal ion has a charge of +1.
For the given options:
Option A: Beryllium is the 4th element of the periodic table having electronic configuration of
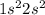
This element will loose 2 electrons to form
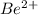 ion.
ion.
Option B: Aluminium is the 13th element of the periodic table having electronic configuration of
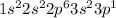
This element will loose 3 electrons to form
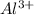 ion.
ion.
Option C: Calcium is the 20th element of the periodic table having electronic configuration of
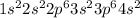
This element will loose 2 electrons to form
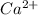 ion.
ion.
Option D: Lithium is the 3rd element of the periodic table having electronic configuration of
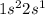
This element will loose 1 electron to form
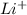 ion.
ion.
Hence, the metal that will form the given compound is lithium.