Answer : 50.69 mg of ascorbic acid does not meet the daily requirement.
Solution : Given,
Molar mass of Ascorbic acid = 176 g/mole
Moles of Ascorbic acid =
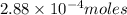
Formula used :
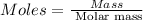
or,
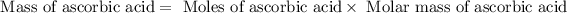
Now put all the given values in this formula, we get the mass of ascorbic acid.
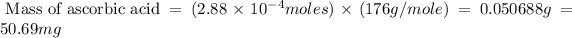
Conversion :
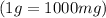
As per question, a healthy adult’s daily requirement of vitamin C is 70-90 mg. But calculate mass of vitamin C is 50.69 mg. So, 50.69 mg of ascorbic acid does not meet the daily requirement.