Boiling point of a compound is determined by the strength of intermolecular forces of attraction between the molecules present in it. Stronger the intermolecular forces of attraction, higher will be the boiling point.
Ionic compounds show ion-ion interactions which are the strongest among all. Ion-dipole interactions are shown when ionic solutes are dissolved in polar solvents. Hydrogen bonding is also a relatively stronger force that is present between H atom and an electronegative atom like F, O and N(
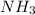 ) . All polar molecules show dipole-dipole interaction (
) . All polar molecules show dipole-dipole interaction (
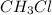 and
and
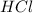 ). Dispersion forces are the weakest intermolecular forces due to momentary dipoles between electron clouds and nucleus.
). Dispersion forces are the weakest intermolecular forces due to momentary dipoles between electron clouds and nucleus.
Among the given compounds,
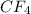 has dispersion forces as the major intermolecular forces of attraction. So they they exhibit the weakest IMF, hence have the lowest boiling point.
has dispersion forces as the major intermolecular forces of attraction. So they they exhibit the weakest IMF, hence have the lowest boiling point.