Answer:- 6.91 kj of heat is needed.
Solution:- We have solid ethanol at -135 degree C and wants to calculate the heat required to convert it to -50 degree C liquid ethanol.
Melting point of ethanol is -114 degree C. So, it is a three step process. In the first step, -135 degree C solid ethanol changes to -114 degree C solid ethanol.
In second step, -114 degree C solid ethanol melts to -114 degree C liquid ethanol. In third step, -114 degree C liquid changes to -50 degree C liquid.
for the first and third step, there is a change in temperature and so we use the equation,
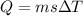
where, Q is the heat energy, m is mass in grams, s is specific heat capacity in joule per gram per degree C and
 is the change in temperature.
is the change in temperature.
For second step, there is a phase change so the equation used is,
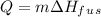
where
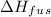 is the enthalpy of fusion.
is the enthalpy of fusion.
Let's do the calculations for the first step:-
 = -114-(-135) = 21 degree C
= -114-(-135) = 21 degree C
m = 25.0 g
s = 0.97 J per g per degree C
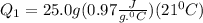
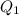 = 509.25 J
= 509.25 J
let's convert this J to kj
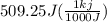
= 0.509 kj
For the second step we need the moles of ethanol as the enthalpy of fusion is given in kj per mol. Molar mass of ethanol is 46.07 g per mol.
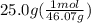
= 0.543 mol
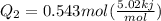
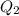 = 2.72 kj
= 2.72 kj
For the third step,
 = -50 -(-114) = 64 degree C
= -50 -(-114) = 64 degree C
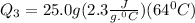
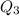 = 3680 J
= 3680 J

= 3.68 kj
total Q =
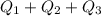
total Q = 0.509 kj + 2.72 kj + 3.68 kj
total Q = 6.909 kj
this could be round to 6.91 kj.
So, 6.91 kj of heat is needed to convert -135 degree C solid ethanol to -50 degree C ethanol.