Answer: D) The lead (II) nitrate is the reaction's limiting reactant.
Step-by-step explanation:
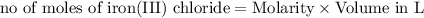
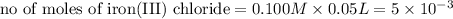
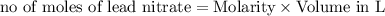
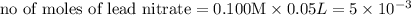
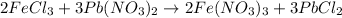
As can be seen from the balanced chemical equation, 3 moles of lead nitrate react with 2 moles of ferric chloride.
Thus
 moles of lead nitrate react with
moles of lead nitrate react with
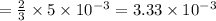 of ferric chloride.
of ferric chloride.
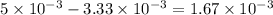 moles of ferric chloride will be left unreacted.
moles of ferric chloride will be left unreacted.
Limiting reagent is the reagent which limits the formation of product. Excess reagent is one which is in excess and thus remains unreacted.
Thus lead nitrate is the limiting reagent and ferric chloride is the excess reagent.