Answer: The roll of lead (II) nitrate is that it is a limiting reactant of the reaction.
Solution : Given,
Molarity of
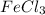 = 0.1 M
= 0.1 M
Volume of
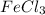 = 50.0 ml = 0.05 L (1 L = 1000 ml)
= 50.0 ml = 0.05 L (1 L = 1000 ml)
Molarity of
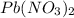 = 0.1 M
= 0.1 M
Volume of
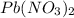 = 50.0 ml = 0.05 L
= 50.0 ml = 0.05 L
First we have to calculate the moles of
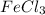 and
and
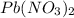 .
.
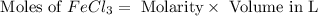
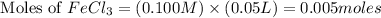
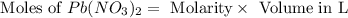
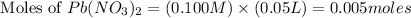
The balanced chemical reaction is,
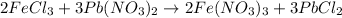
From the balanced chemical equation, we conclude that
3 moles of lead nitrate react with 2 moles of ferric chloride.
Thus 0.005 moles of lead nitrate react with
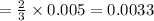 moles of ferric chloride.
moles of ferric chloride.
Moles of ferric chloride will be left unreacted = 0.005 - 0.0033 =0.0017 moles
Limiting reagent is the reagent in the reaction which limits the formation of product.
Excess reagent is the reagent in the reaction which is in excess and thus remains unreacted.
Therefore, in the given reaction, lead nitrate is the limiting reagent and ferric chloride is the excess reagent.