Answer: The correct option is A.
Explanation: Electron affinity is the amount of energy required when an electron is added to a gaseous neutral atom to form a negative ion. This is expressed in kJ/mol.
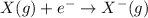
From the given options:
Option A: Negative ion is called as the anion. Electron affinity is the energy required. So, this relates electron affinity and ion formation.
Option B: Positive ion is called as a cation and it is formed by losing an electron. Electron affinity is the energy which is required to add an electron. Hence, this does not relate.
Option C: An isotope is formed when there is a change in the mass number and adding an electron will not affect the mass number. Hence, this does not relate.
Option D: This also does not relate because it is saying that discharge of electrons taking place, which is no true for electron affinity.