Answer: Octet rule
Explanation: The elements which have filled s and p orbitals or noble gases are most stable and thus every element tends to get stable by completing their octet.
For example: Sodium [Na] has atomic no of 11 and thus the electronic configuration is :
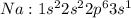 tends to get stable by losing one electron and gets converted to
tends to get stable by losing one electron and gets converted to
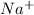
Electronic configuration for
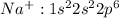
Thus acquiring stable configuration with filled s and p orbitals.
For example: Fluorine [F] has atomic no of 9 and thus the electronic configuration is :
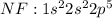 tends to get stable by gaining one electron and gets converted to
tends to get stable by gaining one electron and gets converted to
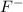
Electronic configuration for
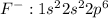
Thus acquiring stable configuration with filled s and p orbitals.