Answer: Option (A) is the correct answer.
Step-by-step explanation:
When there is an increase in oxidation state or release of electron from an atom then this process is known as oxidation.
In the reaction,
 , the oxidation and reduction equations are as follows.
, the oxidation and reduction equations are as follows.
Reduction:
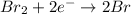
Oxidation:
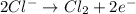
Also, in the above reaction, oxidation state of chlorine increases from -1 to 0. Therefore, chlorine is oxidized in this reaction.
In the reaction,
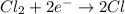 , the oxidation state of chlorine reduces from 0 to -1. Therefore, chlorine is reduced in this reaction.
, the oxidation state of chlorine reduces from 0 to -1. Therefore, chlorine is reduced in this reaction.
In the reaction,
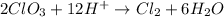 , the oxidation state of chlorine reduces from +6 to 0. Therefore, chlorine is reduced in this reaction.
, the oxidation state of chlorine reduces from +6 to 0. Therefore, chlorine is reduced in this reaction.
In the reaction,
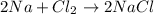 , the oxidation state of chlorine reduces from 0 to -1. Therefore, chlorine is reduced in this reaction.
, the oxidation state of chlorine reduces from 0 to -1. Therefore, chlorine is reduced in this reaction.