Answer: 1.16 moles which contains
 numbers of atoms.
numbers of atoms.
Step-by-step explanation:
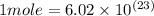
This number represents the number of atoms or molecules in one mole of a compound or a substance.
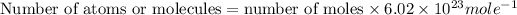
So, if we have
 atoms then the number of moles will be:
atoms then the number of moles will be:
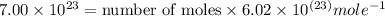
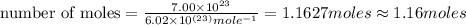
Hence, 1.16 moles which contains
 numbers of atoms.
numbers of atoms.