Step-by-step explanation:
The total valence electrons present in
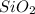 molecule are as follows.
molecule are as follows.
Valency of Si + (Valency of oxygen × number of oxygen atoms)
= 4 + (6 × 2)
= 4 + 12
= 16
Therefore, there are total 16 valence electrons in
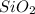 molecule including the ones used to form bonds.
molecule including the ones used to form bonds.