Answer:
The smallest sample size required to obtain the desired margin of error is of 17.
Explanation:
We have that to find our
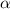 level, that is the subtraction of 1 by the confidence interval divided by 2. So:
level, that is the subtraction of 1 by the confidence interval divided by 2. So:
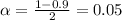
Now, we have to find z in the Ztable as such z has a pvalue of
 .
.
So it is z with a pvalue of
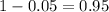 , so
, so
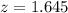
Now, find the margin of error M as such
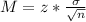
In which
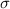 is the standard deviation of the population and n is the size of the sample.
is the standard deviation of the population and n is the size of the sample.
Which of these is the smallest approximate sample size required to obtain the desired margin of error?
The desired margin of error is 20, so
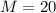
We have that
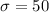 .
.
The smallest sample size is n. So
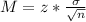
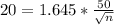
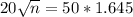
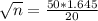
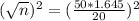
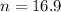
Rounding up
The smallest sample size required to obtain the desired margin of error is of 17.