Answer: Reaction will move in forward direction in order to attain an equilibrium.
Step-by-step explanation:
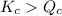 reaction will move in forward direction.
reaction will move in forward direction.
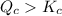 reaction will move in backward direction.
reaction will move in backward direction.
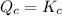 reaction is at equilibrium.
reaction is at equilibrium.
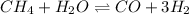
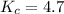
![[H_2O]=0.035 M,[CH_4]=0.050 M,[CO]=0.15 M,[H_2]=0.20 M](https://img.qammunity.org/2019/formulas/chemistry/high-school/ih7ys3zu9o69fpc0d2jm9kh62hrqactwv3.png)
The expression for equilibrium quotient is written as :
![Q_(c)=([CO][H_2]^3)/([H_2O][CH_4])=(0.15* (0.20)^3)/(0.035* 0.050)=0.6857](https://img.qammunity.org/2019/formulas/chemistry/high-school/dlhvimeh75acjxhh5r3ghd0cz8eq4h7f30.png)
Since,
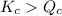 reaction will move in forward direction.
reaction will move in forward direction.