Answer: The pOH of the nitric acid solution is 10.75.
Step-by-step explanation:
The pH of nitric acid solution used by chemist ,
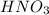 = 3.75
= 3.75
The sum of pH and pOH is always equal to 14 at room temperature.
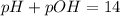
pOH =14 - pH = 14 - 3.72 = 10.75
Hence, the pOH of the nitric acid solution is 10.75.