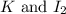Answer: The chemical symbols completing the equation is
Step-by-step explanation:
Decomposition reaction is defined as the reaction in which a single compound gets decomposed to two or more compounds.
General equation representing decomposition reaction follows:
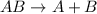
The chemical equation for the decomposition of potassium iodide follows:
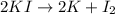
By Stoichiometry of the reaction:
2 moles of potassium iodide decomposes to form 2 moles of potassium element and 1 mole of iodine gas.
Hence, the chemical symbols completing the equation are