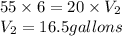Answer: 16.5 gallons of water should be mixed to make a 20% solution of
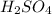
Step-by-step explanation: We are given the concentration of the solution in percentage, it can be taken as the molarity of the solution.
Now, to calculate the volume of water required, we use the formula:
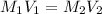
where,
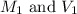 are the initial molarity and volume of the solution.
are the initial molarity and volume of the solution.
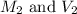 are the final molarity and volume of the solution.
are the final molarity and volume of the solution.
Given:
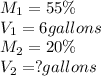
Putting values in above equation, we get: