Answer
35%
Explanation
To answer this, we need to find the weight of the resulting alloy and the weight of the iron in the resulting alloy; then find what fraction of the weight is the weight of the iron in the alloy. and multiply the result by 100% to express it as percentage. Let's do it:
- First, we are going to find the total weight of the resulting alloy:
We know that 4 mg and 12 mg are melted to form the resulting alloy, so
weight of the resulting alloy: 4 mg + 12 mg = 16 mg
- Second, we are going to find the weight of the iron in the resulting alloy:
We know that the metal alloy weighing 4 mg contains 20% iron, so to find the weight of iron in it, we need to find the 20% of 4 mg:
20% of 4 mg =
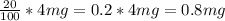
Similarly, the alloy weighing 12 mg contains 40% iron, so let's find the weight of the iron in it:
40% of 12 mg =
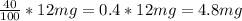
Now, we just need to add the weights:
weight of the iron in the resulting alloy: 0.8 mg + 4.8 mg = 5.6 mg
- Finally, to find what fraction of the weight is the weight of the iron in the alloy, we just need to divide the weight of the iron in the resulting alloy by the weight of the resulting alloy:
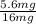 *100% = 35%
*100% = 35%
We can conclude that 35% of the resulting alloy is iron.