Answer : The correct option is, There are two nitrate ions.
Explanation :
Calcium nitrate is formed by the combination of a metal cation and poly-atomic anion. On the dissociation, it will dissociates into one calcium ion and two nitrate ions.
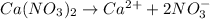
Hence, the subscript, 2 indicates that there are two nitrate ions in calcium nitrate.