Answer: A. hydrogen gas and zinc chloride
Step-by-step explanation: Reactants of a chemical reaction is defined as the substances which take part in a chemical reaction and undergoes a chemical change. Reactants are written on left side of the arrow.
Products are defined as the substance which are formed at the end of the chemical reaction.Products are written on the right side of the arrow.
For the reaction of zinc (Zn) and hydrochloric acid (HCl), the balanced chemical equation is represented as :
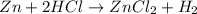
Here,
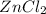 and
and
 are products.
are products.