Answer : The number of moles present in barium nitrate solution is, 0.50 moles
Solution : Given,
Molarity of barium nitrate solution = 2 M = 2 mole/L
Volume of solution = 0.25 L
Molarity : Molarity is defined as the number of moles of solute present in one liter of solution.
Formula used :
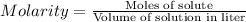
or,
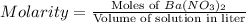
Now put all the given values in this formula, we get the moles of barium nitrate.
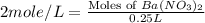
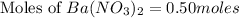
Therefore, the number of moles present in barium nitrate solution is, 0.50 moles