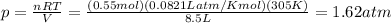Answer:
1.62 atm
Step-by-step explanation:
We can solve the problem by using the ideal gas equation:
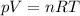
where:
p = ? is the pressure of the gas in the tire
V = 8.5 L is the volume of the tire
n = 0.55 mol is the number of moles of the gas
R = 0.0821 atm L / K mol is the gas constant
T = 305 K is the temperature of the gas
By re-arranging the equation and substituting the numbers in, we find: