Answer: 1)
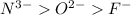 ; the effective nuclear charge is smallest at
; the effective nuclear charge is smallest at
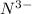 and the largest at
and the largest at
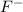
Explanation:
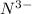 : Atomic number or number of electrons or number of protons in Nitrogen is 7. As
: Atomic number or number of electrons or number of protons in Nitrogen is 7. As
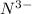 carries extra three electrons, the total no of electrons is 7+3=10.
carries extra three electrons, the total no of electrons is 7+3=10.
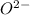 : Atomic number or number of electrons or number of protons in Oxygen is 8. As
: Atomic number or number of electrons or number of protons in Oxygen is 8. As
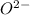 carries extra two electrons, the total no of electrons is 8+2=10.
carries extra two electrons, the total no of electrons is 8+2=10.
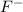 : Atomic number or number of electrons or number of protons in Fluorine is 9.
: Atomic number or number of electrons or number of protons in Fluorine is 9.
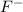 carries extra one electron, the total no of electrons is 9+1=10.
carries extra one electron, the total no of electrons is 9+1=10.
Thus all the three species contain 10 electrons and thus are called as iso electronic species.
7 protons of nitrogen will not be able to hold 10 electrons tightly and thus the electrons will remain loosely bound, the effective nuclear charge will be least and the size will be largest. Whereas 10 protons of fluorine will effectively hold 10 electrons towards itself, the effective nuclear charge is highest and leads to smallest size.