Answer: The four
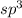 hybridized orbitals on Sb makes up the sigma bonds between Sb and F in antimony(iii) fluoride ,
hybridized orbitals on Sb makes up the sigma bonds between Sb and F in antimony(iii) fluoride ,
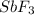
Step-by-step explanation:
According to VESPR theory:
Number of electrons around the central atom :
![(1)/(2)[V+N-C+A]](https://img.qammunity.org/2019/formulas/chemistry/college/behunmw9o38v8waaz7fjxke5kwwn5ol1qv.png)
V = number of valence electrons
N = number of neighboring atoms
C = charge on cation
A = charge on an anion
In antimony(III) fluoride ,
Antimony being central atom: V= 5,N =3,C=0,A=0
Number of electrons :
![(1)/(2)[V+N-C+A]=4](https://img.qammunity.org/2019/formulas/chemistry/college/qzry23zpzka3cfypw7yk2ehbxt5bruv3ta.png)
Number of electrons around the central atom are 4 which means that
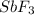 molecule has four
molecule has four
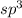 hybridized orbitals.
hybridized orbitals.