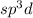Answer : The correct answer is, Valence electrons.
Step-by-step explanation:
Lewis-dot structure : It is also known as electron-dot structure. It shows the bonding between the atoms of a molecule. it also shows the unpaired electrons present in the molecule.
When we are determining the shape of a molecule then it is important to draw a Lewis-dot structure and for drawing a Lewis-dot structure we should know that how many total number of valance electrons present in a given compound or a molecule.
For example :
The given molecule is,
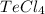
As we know that tellurium has '6' valence electrons and chlorine has '7' valence electron.
Therefore, the total number of valence electrons in
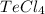 = 6 + 4(7) = 34
= 6 + 4(7) = 34
In
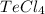 , one-one electron of 4 chlorine combine with 4 electrons of tellurium and 2 pair electrons of tellurium left as a lone pair. That means, it has 4 bonding pairs and 1 lone pairs. So, the hybridization will be,
, one-one electron of 4 chlorine combine with 4 electrons of tellurium and 2 pair electrons of tellurium left as a lone pair. That means, it has 4 bonding pairs and 1 lone pairs. So, the hybridization will be,